Scientists Propose IAP Process for Separation of Aluminum Alloys
Professor SHI Weiqun's group from the Institute of High Energy Physics, Chinese Academy of Sciences, has proposed a modified electrorefining process to recover actinides, namely in-situ anodic precipitation (IAP). This study, entitled "In-situ Anodic Precipitation Process for Highly Efficient Separation of Aluminum Alloys", was published online in Nature Communications.
Shi's group has long been committed to basic research on the dry reprocessing of spent nuclear fuels. In order to improve the separation efficiency of actinides over lanthanides, a new concept of efficient separation and recovery of actinides based on a solid active aluminum cathode has been developed in recent years. However, the cathode product will be aluminum alloy if a solid aluminum cathode is utilized to recover actinides and it cannot be directly reused in fuel manufacturing. As a result, further separation of actinides from aluminum is necessary. Current separation methods involving aluminum alloys are usually quite complicated and thus greatly restrict the practical application of solid aluminum cathode-based separation technology.
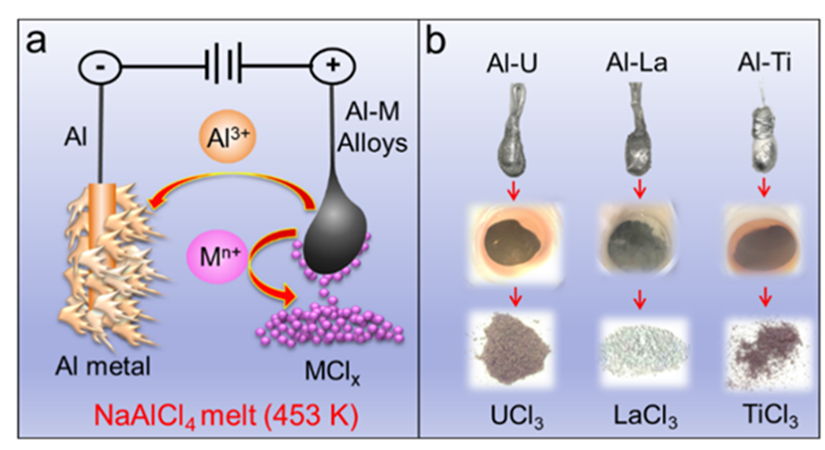
Recently, Shi's group proposed a new strategy for separating actinide aluminum alloys based on different solubilities of target metal chlorides in the NaAlCl4 molten salt. This method is called the “in-situ anodic precipitation process.” NaAlCl4 molten salt was selected as the electrolyte and a uranium aluminum alloy and an aluminum rod were selected as the anode and cathode, respectively (Fig. 1a). Insoluble UCl3 in NaAlCl4 molten salt was generated at the anode after anodic oxidation and then was recovered in the form of precipitation at the bottom of the electrochemical cell (Fig. 1b).
This new method is a simple and practical means for the recovery of actinides from aluminum alloys, and is also a convenient synthetic approach for metal chlorides in low oxidation states.
The project was supported by the National Science Fund for Distinguished Young Scholars and the Major Program of the National Natural Science Foundation of China.
Contact Information
Mr. GUO Lijun
ljguo@ihep.ac.cn