Scientists Propose Nanocluster-based Actinide Separation Method
Professor Shi Weiqun's group from the Institute of High Energy Physics, Chinese Academy of Sciences, recently proposed a new nanocluster-based paradigm for actinide separation, namely nano-extraction. This brand new strategy can achieve efficient sequestration of uranium in the unprecedented form of giant coordination nanocages. The study, entitled "Actinide Separation Inspired by Self-Assembled Metal-Polyphenolic Nanocages," was recently published in the Journal of the American Chemical Society.
The separation of actinides has a vital place in nuclear fuel reprocessing, recovery of radionuclides and remediation of environmental contamination. Traditional actinide extraction is achieved through selective complexation of actinide cations from mixtures containing multiple metal ions by exquisitely designed organic extractants. The use of large cluster compounds for actinide separation has rarely been pursued. In contrast to simple complexes, such assemblies, especially metal-organic coordination assemblies with nanometer-scale dimensions, impose strict requirements on both molecular geometries of the constituent ligands and their coordination preference for targeted metal ions, an effect termed "multivalent cooperativity" (Fig. 1a). Such cooperativity can lead to highly selective formation of exquisite multinuclear assemblies by efficiently excluding undesired ions. Another attribute of this approach is that multivalent interactions can result in remarkable enhancement of the stability of the supramolecular architectures produced, thus facilitating both extraction and sequestration.
In this study, a novel strategy for efficient separation of actinides by well-defined metal-polyphenolic nanocages, which are assembled in situ from uranyl and cone-shaped macrocyclic pyrogallol[4]arenes, is proposed. The U24-based hexameric pyrogallol[4]arene nanocages with distinctive [U2PG2] binuclear units (PG = pyrogallol) that rapidly assembled in situ in monophasic solvent were identified by single-crystal x-ray diffraction (Fig. 1b), MALDI-TOF mass spectrometry, nuclear magnetic resonance spectroscopy, and small-angle X-ray/neutron scattering techniques (Fig. 1c, d). Notably, the [U2PG2] structural unit is quite disparate from the trinuclear [M3PG3] moiety that is ubiquitous in other metal-seamed hexameric pyrogallol[4]arene nanocapsules. This difference might reflect the larger effective radius and distinct coordination behavior of the uranyl moiety versus bare transition metals, as well as main group and alkaline earth metals. Comprehensive biphasic extraction studies show that this novel separation strategy has enticing advantages such as fast kinetics, with equilibrium attained within 30 min, high efficiency, and good selectivity for lanthanides.
This type of extraction as nanoscale species represents a novel paradigm for hybrid nanocluster-based actinide sequestration and may be considered "nano-extraction." Future efforts in this realm should optimize ligand design for improved efficiency and better adaptability, as well as utility in real-world separations such as the separation of U from other actinides such as Np and Pu.
This project was supported by the National Science Fund for Distinguished Young Scholars, the General Program of the National Natural Science Foundation of China and the Youth Innovation Promotion Association of CAS.
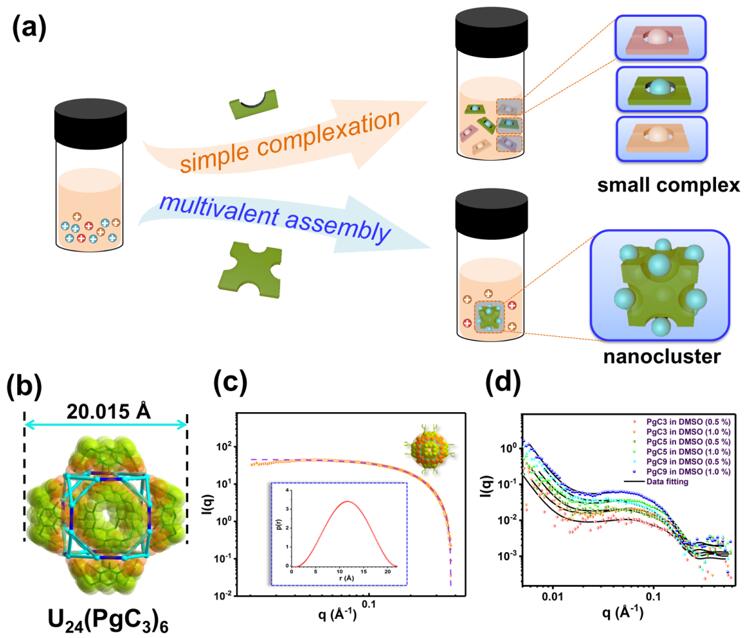
Fig. 1. Nano-extraction of actinides based on in situ assembled U24-based hexameric pyrogallol[4]arene nanocages
Contact Information
Mr. Guo Lijun
ljguo@ihep.ac.cn