Scientists Develop a Potential Cancer Theranostic Tool Using Catalytic Analysis
A new strategy for constructing an artificial metalloenzyme for cancer theranostics by efficiently analyzing the enzyme's catalytic mechanism has been recently developed by Prof. Xueyun Gao's group from the Beijing University of Technology and Prof. Lina Zhao's group from the Institute of High Energy Physics. The study, entitled "An artificial metalloenzyme for catalytic cancer-specific DNA cleavage and operando imaging," was published in Science Advances.
This study describes the design and synthesis of an artificial metalloenzyme using theoretical calculations and quantitative experiments. The artificial metalloenzyme in this research consists of one copper cluster firmly anchored in bovine serum albumin conjugated with a tumor-targeting peptide. The artificial metalloenzyme has an appropriate energy level position and a selectively flexible geometrical structure that allow it to persistently catalyze the H2O2-decomposition reaction in the tumor microenvironment to produce hydroxyl radicals (●OH) and oxygen. This reaction is beneficial because freely diffusible ●OH is one of the intermediates involved in the DNA scission process and finally induces tumor cell apoptosis. In addition, the sustainable and sensitive chemiluminescence imitated by ●OH enables real-time tracing and evaluation of tumor therapy in situ (Fig. 1).
The research reveals key aspects of the artificial metalloenzyme, including its fine molecular structure, detailed electronic parameters and intrinsic enzyme mechanisms. It shows that the relative position of the clusters’ energy levels, which produces the inherent quantum size effect, is facilely tuned to catalyze the specific reaction mentioned above. Notably, during the catalytic process, the metal valence state of the metalloenzyme is efficiently recycled, ensuring that the catalytic active centers are not consumed, and a stable and persistent catalysis recycling system exists (Fig. 2).
These findings provide new guidance, both from a structural and functional viewpoint, for the rational design and precise synthesis of biocompatible metalloenzymes on demand. This study also provides new insights on visually monitoring and efficiently combating specific cancers by exploring the distinctive catalytic activities of metal clusters.
Artificial metalloenzymes mimic natural metalloenzymes to achieve many key cutting-edge catalytic applications. Clearly understanding the catalytic mechanism underlying the effective function of artificial metalloenzymes will certainly boost the rational design of artificial metalloenzymes with specific characteristics.
Article Link: https://advances.sciencemag.org/content/6/29/eabb1421

Figure 1. Schematic diagram of a protein-cloaked copper cluster acting as an artificial metalloenzyme with persistent catalytic activity for high-efficient DNA cleavage and operando chemiluminescent imaging in the tumor microenvironment.
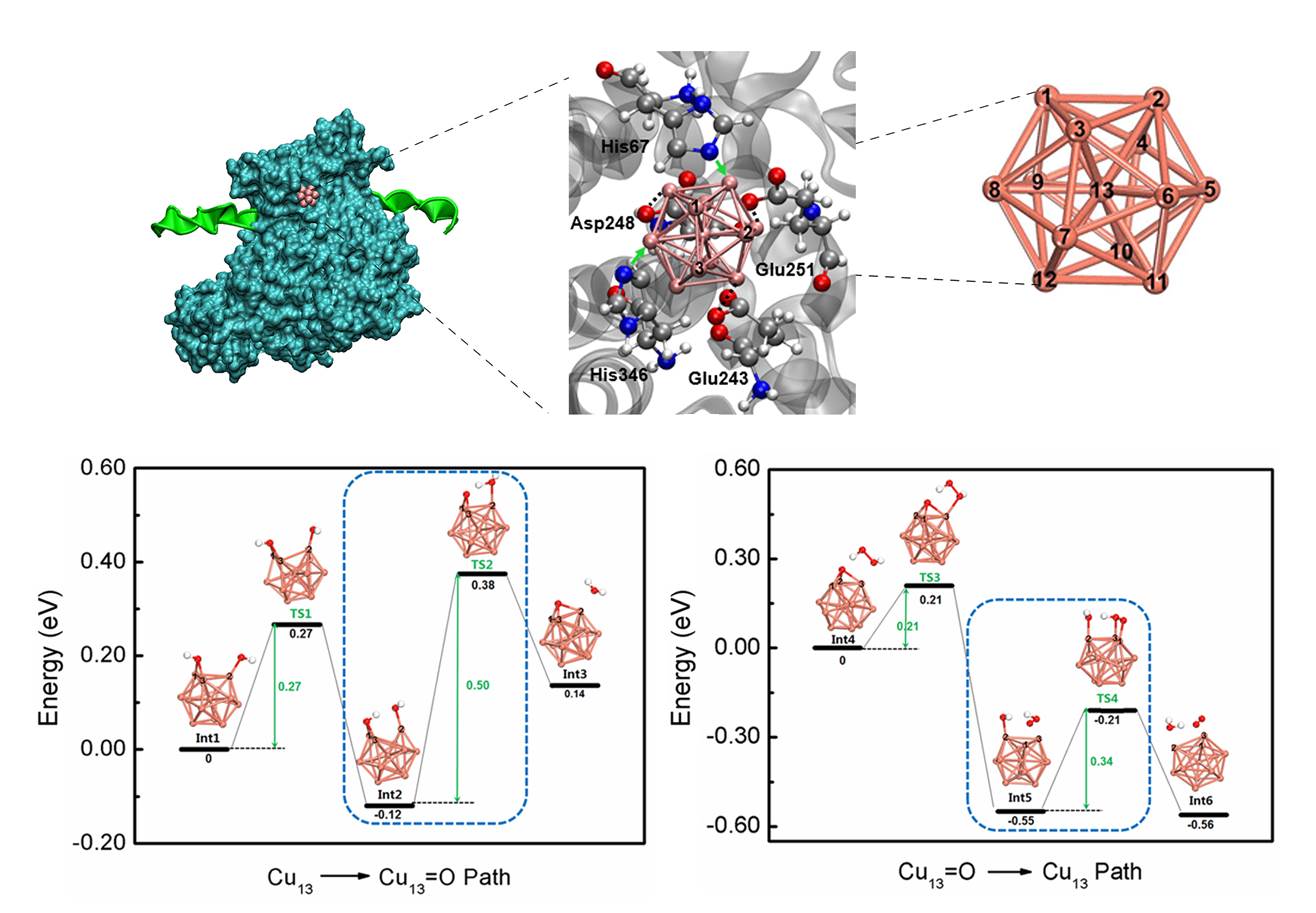
Figure 2. The artificial metalloenzyme’s fine molecular structure and reaction pathways of the recycled catalytic mechanism.
Contact Information
Mr. Guo Lijun
ljguo@ihep.ac.cn