Development of Novel Cell-targeted Strategies for Improved Anticancer Efficacy
Small-molecule drugs typically have a poor capacity for specifically targeting tumor cells, thus diminishing the therapeutic efficacy of such drugs. To combat this problem, cell-targeted drug delivery strategies are needed that will facilitate the accumulation of drugs in the tumor.
Stimuli-responsive nanoparticles are a promising means of targeted drug delivery. They can release anticancer drugs based on endogenous tumor-specific conditions (e.g., low pH, reducing substances, etc.) or external stimuli (e.g., light, ultrasound, etc.) at the tumor site. Over the past few years, many researchers have explored their potential.
Researchers at the Institute of High Energy Physics (IHEP) have been part of this effort. We have recently found a remarkable difference in mitochondrial temperature between cancer cells and normal cells, including macrophages and human umbilical vein endothelial cells.
By taking advantage of this temperature difference, they developed a mitochondria-targeted thermoresponsive drug carrier that is able to release considerably more drugs in cancer cells than normal cells (Chem. Commun., 2019, 55, 4051). Therefore, mitochondrial temperature might be a novel tumor-specific condition for developing tumor-targeted drug delivery.
Besides endogenous tumor-specific conditions, they also harnessed physical means as external stimuli for tumor targeting. For instance, light can induce drug release at the tumor in a spatiotemporal fashion. We have developed near-infrared (NIR) light-responsive nanoparticles that enhance the anticancer efficacy of drugs in both subcutaneous and orthotopic tumor models (ACS Appl. Mater. Interfaces, 2016, 8, 15103; ACS Biomater. Sci. Eng., 2017, 3, 3628).
Recently, IHEP scientists and collaborators have further developed NIR light-responsive optoelectronic material for photothermal/photodynamic synergistic therapy (ACS Appl. Mater. Interfaces, 2019, 11, 17884). These studies indicate that NIR light can be a powerful tool for tumor targeting.
Nevertheless, tumor-specific drug release might not be sufficient to eradicate solid tumors, due to low tumor penetration. Although decoration with polymers (e.g., PEG) increases the stability of nanoparticles in circulation, it inevitably hinders the tumor penetration of drugs. To circumvent the “PEG dilemma”, scientists developed an NIR light-responsive dePEGylation strategy. Specifically, the embedded upconversion nanocrystals effectively convert NIR to UV-visible light, which can cleave the linker between PEG and nanoparticles. Therefore, by exposing the tumor region to NIR light, PEG is removed from the nanoparticles, thus facilitating the deep penetration of drugs into the tumor. These results were published in Nano Letters (DOI: 10.1021/acs.nanolett.9b00737).
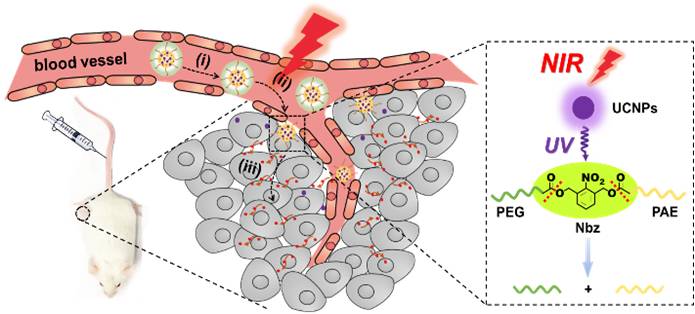
Figure: Illustration of NIR-guided nanodrugs surmounting physiological barriers (i, blood circulation; ii, extravasation; iii, tumor penetration).
Contact Information
Mr. Guo Lijun
ljguo@ihep.ac.cn