| A lattice-oxygen-involved reaction pathway boosting urea oxidation |
| From: PublishDate:2020-07-29 Hits: |
Urea oxidation reaction (UOR) is known as a central reaction in the area of clean energy storage and conversion, urea fuel cells, de-ureation of wastewater, artificial kidney and etc. However, UOR suffers from its sluggish reaction kinetics in the conventional UOR mechanisms, which greatly impedes its practical applications. A research team (Peng group) from Fudan University, for the first time, has reported a novel lattice-oxygen-involved UOR mechanism, which has faster reaction kinetics than the conventional UOR mechanisms. Their research was published on October 11th, 2019 in Angewandte Chemie, International Edition. Previous studies showed that nickel oxides exhibited higher catalytic activity for UOR than other metals and metal oxides in alkaline media, where Ni3+ sites were identified as the catalytic active sites. Besides, due to the unfavorably strong binding of Ni3+ sites with the *COO intermediates, the CO2 desorption step was proven as the rate-determining step (RDS). Breakthroughs in facilitating the CO2 desorption in RDS are desired to develop high-performance UOR catalysts. Combining density functional theory studies, 18O isotope-labeling mass spectroscopy and in-situ infrared spectroscopy, we find that lattice oxygen is directly involved in transforming *CO to CO2 and accelerating the UOR rate. The resultant Ni4+ catalyst on glassy carbon electrode exhibits a high current density of 264 mA/cm2 at 1.6 V versus reversible hydrogen electrode, and the turnover frequency of Ni4+ active sites towards UOR is 5-times higher than that of Ni3+ active sites.
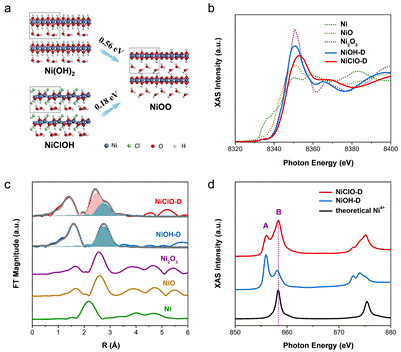
The electronic structure of Ni4+ (NiClOH-derived, NiClO-D) and Ni3+ (Ni(OH)2- derived, NiOH-D) catalysts were probed and confirmed using X-ray absorption fine structure spectroscopy measurements at 1W1B-XAFS station of Beijing Synchrotron Radiation Facility (BSRF). The higher oxidation state of Ni active sites in Ni4+ catalyst and its endowing enhanced Ni-O covalency, trigger the activation of lattice oxygen to couple with *CO intermediate, and thus enable a lattice-oxygen-involved reaction pathway. The increased oxidation state of metal sites not only governs the catalytic activity but also the reaction mechanism, where lattice oxygen can be activated and promote new reaction pathways. Article: Longsheng Zhang, Liping Wang, Haiping Lin, Yunxia Liu, Jinyu Ye, Yunzhou Wen, Ao Chen, Lie, Wang, Fenglou, Ni, Zhiyou Zhou, Shigang Sun, Youyong Li*, Bo Zhang* and Huisheng Peng*. A lattice-oxygen-involved reaction pathway boosting urea oxidation. Angew. Chem. Int. Ed., 2019, 58, 16820. |
|
|
| Chinese
- Metal-free efficient photocatalyst for stable visible water splitting——Top ten major scientific progresses in China in 2015
- The nano-resolution imaging platform was awarded the first rate prize of Beijing Science and Technology in 2014
- Beamline 1W1 of BSRF started to runoperate in the couplingparasitic mode of BEPCII
- Synthesis of High Performance Polymer Materials for Field Effect-Transistors
- Surfactant molecular aggregates in green solvents
- GIXRD has played an important role in the characterization of organic thin-film transistors
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility