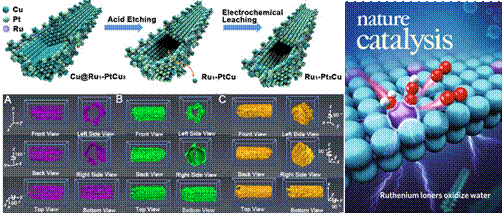
| Highly efficient single atom Ru catalyst boosts acidic water electrolysis |
| From: PublishDate:2020-07-24 Hits: |
The production of hydrogen energy is mainly by methane reforming in the industry, accompanied with the release of greenhouse gas CO2, which is environmentally unfriendly. However, producing hydrogen from water splitting is feasible and without carbon emission, which is expected to replace “methane steam reforming” and become the next generation of hydrogen technology. Currently, the biggest challenge of the hydrogen production from water splitting is the cost. In the industry, the cost of hydrogen production from water splitting is 4.7-5.4 $/kg. The cost of hydrogen production from methane reforming is 2.7-3.1 $/kg. Therefore, realizing the production of hydrogen by water splitting replace of methane reforming in the future, we must develop cheap and efficient catalysts to reduce the cost of water splitting. The research group of Professor Wu Yuen of the University of Science and Technology of China (USTC) successfully prepared single atom Ru catalyst by surface defect engineering approach. The single atom Ru catalyst delivers 90mV lower overpotential to reach a current density of 10 mA cm-2, and an order of magnitude longer lifetime over that of commercial RuO2. This work provides a new idea to solve Ru-based catalysts unstable under acidic oxidation conditions, which is an international problem. Related research was published in Nature Catalysis with the title of “Engineering the electronic structure of single atom Ru sites via compressive strain boosts acidic water oxidation electrocatalysis” and selected as cover paper.
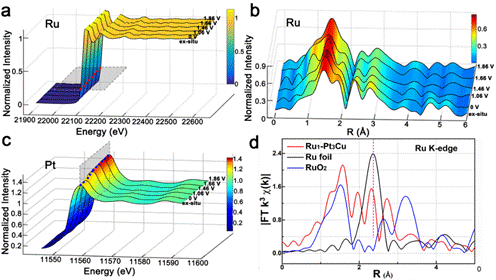
Figure 1. The Schematic diagram of the preparation of single atom Ru catalyst and the cover of the article. In this work, the electronic structure and coordination environment of the substrate Pt and Cu were studied utilizing the 1W1B-XAFS station of Beijing Synchrotron Radiation Facility (BSRF). The X-ray absorption near-edge structure (XANES) and extended x-ray absorption fine structure of Pt revealed that Pt existed in a metallic state and the main coordination bonds were Pt-Pt/Cu. The analysis of XANES of Cu element showed that the valence of Cu was between 0~2. The catalytic mechanism of the catalyst was further explored by in-situ synchrotron radiation. For Pt, with the potential scanned from 0 to 1.86 V, the valence state of Pt increased, as shown by the white line intensity of the Pt L3-edge. This indicates that there exists electrons transferring from Pt to Ru1, avoiding over-oxidation of Ru species.
Figure 2. (a-c) In-situ and (d) ex-situ XAFS spectrum of Ru single atom. Article: Yancai Yao, Sulei Hu, Wenxing Chen, Zheng-Qing Huang, Weichen Wei, Tao Yao, Ruirui Liu, Ketao Zang, Xiaoqian Wang, Geng Wu, Wenjuan Yuan, Tongwei Yuan, Baiquan Zhu, Wei Liu, Zhijun Li, Dongsheng He, Zhenggang Xue, Yu Wang, Xusheng Zheng, Juncai Dong, Chun-Ran Chang, Yanxia Chen, Xun Hong, Jun Luo, Shiqiang Wei, Wei-Xue Li*, Peter Strasser, Yuen Wu* and Yadong Li, Engineering the electronic structure of single atom Ru sites via compressive strain boosts acidic water oxidation electrocatalysis, Nature Catalysis, 2019, 2, 304–313. |
|
|
| Chinese
- Metal-free efficient photocatalyst for stable visible water splitting——Top ten major scientific progresses in China in 2015
- The nano-resolution imaging platform was awarded the first rate prize of Beijing Science and Technology in 2014
- Beamline 1W1 of BSRF started to runoperate in the couplingparasitic mode of BEPCII
- Synthesis of High Performance Polymer Materials for Field Effect-Transistors
- Surfactant molecular aggregates in green solvents
- GIXRD has played an important role in the characterization of organic thin-film transistors
Science Highlights
Home /
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility