| The study of an unusual 32-membered copper(II) metallomacrocube |
| From: PublishDate:2017-06-16 Hits: |
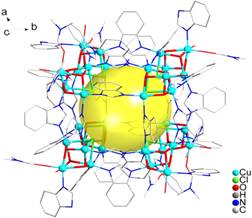
The synthesis of large metal-containing clusters is of considerable interest because of their promising applications in many fields such as catalysis, gas storage, magnetism, and molecular recognition on account of their high nuclearity. Cu-based polynuclear complexes have attracted spectacular attention because they are good candidates for exploring magneto-structural correlations resulting from the mutual interactions among the metal centers, and Cu is an essential bioelement in charge of numerous catalytic processes in living bodies. Several Cu-based polynuclear materials have been successfully synthesized. Nevertheless, polycopper(II) cluster complexes with a higher number of Cu atoms have been rarely reported. To date, only 43 examples of Cu-based discrete clusters have been known with nuclearity ≥30. The selection of modules and rational self-assembly are the main problems. The Cu4X4 (X = anion or dianion) cubane framework is one of the most popular modules for Cu-based complexes. Nevertheless, polycopper(II) clusters featuring a multi-cubane structure are limited in number. Only two compounds based on the Cu4X4 cubical core framework with nuclearity ≥10 have been reported. In this paper, the structure and properties of the first 32-membered Cu(II) cluster based on a Cu4O3X cubic core [(Cu4Cl(OH)3)8(BI)24(HBI)6(H2O)3.5(DMF)4.5]8+·8OH-·4H2O·3.5DMF (HBI = benzimidazole) have been reported. Single-crystal X-ray diffraction data for the complex were collected in Beijing Synchrotron Radiation Facility (BSRF) beam line 3W1A which mounted with a MARCCD-165 detector (λ = 0.72000 ? ) with storage ring working at 2.5 GeV. In the process, the crystals were protected by liquid nitrogen at 100(2) K. The X-ray structural analyses show that the complex crystallizes in the trigonal space group R-3 as a discrete 32-nuclear cationic cluster with hydroxide counterions. The unprecedented 32-nuclear cation Cu cluster can be described as eight cuboidal [Cu4O3Cl] tetramers, bound together with peripheral BI- ligands, resulting in a giant metallomacrocube. The maximum diameter of the [Cu32] cluster was ~18 ? , with a shortest intercluster center-to-center distance of ~9 ? . For the cuboidal [Cu4O3Cl] tetramer, the Cu atoms are located at the vertices of a distorted cube, and in each cube, four Cu atoms are held together tightly by three μ3-hydroxido oxygen atoms and one μ3-chlorido. The intracube Cu…Cu distances are 3.102–3.263 ? , indicating potential moderate magnetic interactions among the Cu atoms. Interestingly, benzimidazole ligands show two different coordination modes. One appears as the wheels of the metallomacrocube from three perpendicular directions including 24 BI- ligands, and the other 6 HBI ligands are terminal groups coordinated to the tetramers. To the best of our knowledge, the Cu32 cluster in this study is the largest Cu cluster based on the Cu4X4 cubical core framework. From the application perspective, the complex exhibited strong antiferromagnetic interactions and excellent catalytic performance in the photocatalytic degradation of organic pollutant rhodamine B under UV light irradiation. Moreover, the complex exhibited good electrocatalytic activity for nitrite reduction.
Fig. 1 View of the 32-nuclear metallomacrocube unit of the complex, hydrogen atoms were omitted for clarity. The vertices of the big cube with a cavity (yellow ball) of ~9 ? diameter were occupied by eight small cubical Cu4O3Cl cores.
Article: An unusual 32-membered copper(II) metallomacrocube based on a Cu4O3X cubic core: photocatalytic, electrocatalytic, and magnetic properties. Chem. Commun., 2016, 52 (23): 4294-7. |
|
|
| Chinese
- Metal-free efficient photocatalyst for stable visible water splitting——Top ten major scientific progresses in China in 2015
- The nano-resolution imaging platform was awarded the first rate prize of Beijing Science and Technology in 2014
- Beamline 1W1 of BSRF started to runoperate in the couplingparasitic mode of BEPCII
- Synthesis of High Performance Polymer Materials for Field Effect-Transistors
- Surfactant molecular aggregates in green solvents
- GIXRD has played an important role in the characterization of organic thin-film transistors
Science Highlights
Home /
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility