| K-supported catalysts for diesel soot combustion: Making a balance between activity and stability |
| From: PublishDate:2017-06-16 Hits: |
Soot particles from the diesel vehicles could lead to the serious environmental and health problems, which is one of the main source of smog. The aftertreatment method by catalysts is one of the most efficient techniques to remove soot particles. Herein, alkali metals (especially K) contianing catalytsts exhibit superior catalytic soot combustion performance, which is hopeful to substitute for commercially used noble metal catalysts. A team from University of Jinan has investigated K-supported oxides (K/Al2O3, K/TiO2) for catalytic soot combustion. The catalytic activity and stability have been studied in detail. A strategy for balancing the activity and stability has been proposed aiming at the trade-off relationship between them. Their research has been published on Catalysis Today in 2016. K/Al2O3 shows two kinds of K species consisting of the dispersed K+--O–CO2 species (free K species) and a K2Al2O4 phase (K+ having been incorporated into Al2O3). However, all the supported K entered into the lattice of TiO2 to form K2Ti6O13 for K/TiO2. K/Al2O3 shows much higher activity for soot combustion. Comparatively, K/TiO2 is hardly active. The active species is confirmed to be the free K+ species. After water washing, the catalytic activity of K/Al2O3 decreased to a large extent due to the loss of free K+ species, leading to the poor water-resistant stability. A strategy for making a balance between activity and stability, two sides of the coin for K-containing catalysts, is encapsulating of active K species in the pore structures of the catalyst, as demonstrated by the tunneled cryptomelane. This tunneled configuration secured free K+ against leaching in water, which opens an efficient pathway to practical usage of K-containing catalysts for soot removal.
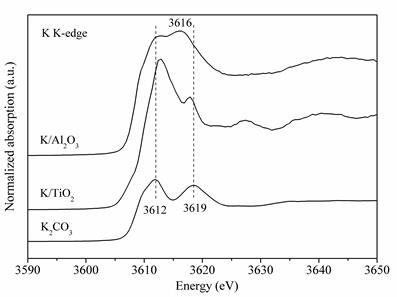
The normalized absorption of K K-edge for K/Al2O3, K/TiO2 and K2CO3 were obtained using synchrotron radiation at BSRF. For K/Al2O3, peaks were located at the similar energy as that for K2CO3, suggesting that the K carbonate species are present in K/Al2O3. Besides, a new peak at 3616 eV appeared, indicating that the coordination state of K was influenced by the presence of Al, which is attributed to the formation of K2Al2O4 from XRD results. However, for K/TiO2, completely different absorption peaks from that of K2CO3 were observed, with the peak at 3612 eV shifting to higher energy while that at 3619 eV shifting to lower energy. This confirmed that in K/TiO2, all supported K2CO3 has been transformed into a new phase K2Ti6O13 according to XRD results. The research investigated the relationship between activity and stability of alkali metal catalysts for soot combustion and thus a strategy to balance them was proposed. Synchrotron sources have helped the team to clarify the changes of potassium species derived from the loading of K on oxides with different electro-negativities. "To obtain alkali metal catalysts with superior activity and high stability for soot combustion, K+ can be encapsulated in the channel of catalyst and thus K+ can be protected. The existential states of K+ should be studied to a further degree to build the relationship between the structure and the catalytic performance of alkali metal catalysts. More information from synchrotron studies would surely help to examine the existential states and structures of alkali metals in the channels of catalysts on a smaller scale." explains by professor Zhaoliang Zhang, the team leader from University of Jinan. Article: Qian Li, Xiao Wang, Hui Chen, Ying Xin, Guangkai Tian, Chenxi Lu, Zhaoliang Zhang*, Lirong Zheng, Lei Zheng, K-supported catalysts for diesel soot combustion: Making a balance between activity and stability. Catalysis Today 264 (2016), 171–179. |
|
|
| Chinese
- Metal-free efficient photocatalyst for stable visible water splitting——Top ten major scientific progresses in China in 2015
- The nano-resolution imaging platform was awarded the first rate prize of Beijing Science and Technology in 2014
- Beamline 1W1 of BSRF started to runoperate in the couplingparasitic mode of BEPCII
- Synthesis of High Performance Polymer Materials for Field Effect-Transistors
- Surfactant molecular aggregates in green solvents
- GIXRD has played an important role in the characterization of organic thin-film transistors
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility