| Solving important protein structures using precipitant-immobilized imprinted polymers |
| From: PublishDate:2016-06-03 Hits: |
A team from China Agricultural University published their research in Nature Communications on March 23th, 2015. The research is about “the amino-terminal structure of human Fragile X Mental Retardation Protein obtained using precipitant-immobilized imprinted polymers”. Flexibility is an intrinsic property of proteins. Few proteins could carry out their biological functions without structural flexibility. Proteins have many flexible components, such as loops, turns, termini and side chains of residues in helices or strands. Unfortunately, the flexibility of proteins makes proteins difficult to grow crystals giving rise to the bottleneck of gaining high-quality protein crystals. Moreover, the traditional protein crystallography methods are seldom successful for highly flexible proteins. Fragile X Mental Retardation Protein (FMRP) was the main factor causing fragile X syndrome, the most common form of inherited mental retardation in humans with a frequency of 1:4000 males and 1:6000 females. However, the structure and molecular mechanism are unclear.
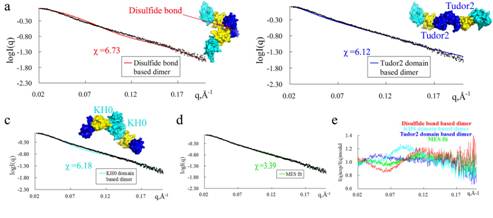
Figure: FMRP has several kinds of dimers in solution. Comparison of experimental data and calculated scattering profiles for wild-type FMRPΔ. Experimental data are represented in black dots. (a) The theoretical scattering curves of disulfide bond-based dimer (red), (b) Tudor2 domain-based dimer (blue), (c) KH0 domain-based dimer (cyan) and (d) the assembly from MES fit (green) are shown. (e) Residuals of four models calculated as I(q)experimental/I(q)model. The team developed an undescribed and intriguing approach that immobilizing common protein precipitants such as polyethylene glycol and ammonium sulfate onto molecularly imprinted polymers to yield high-quality single crystals after four years’ experiments on FMRP. Only by using this method, high-quality single crystal of a structure-uncharacterized segment of Fragile X Mental Retardation Protein was successfully obtained, and structure was solved. Besides, a novel KH domain-KH0 and an intermolecular disulfide bond were identified for the first time. Moreover, the precipitant-immobilized molecularly imprinted polymers (piMIPs) significantly increased the diffraction resolution limit compared to other conventional nucleants for five model proteins. So the piMIPs materials might be widely used in protein crystallization, especially for flexible proteins. The team performed the small angle X-ray scattering analysis (SAXS) experiments at the BioSAXS station (1W2A) of the BSRF. SAXS is a powerful tool for structure validation and the quantitative analysis of flexible systems, and is highly complementary to the high resolution methods of X-ray crystallography and NMR. After carefully analyzing the component of the dimeric components in solution, it was found that FMRP has several kinds of dimeric conformations in solution, providing insights into the function of this protein. Article: Yufeng Hu, Zhenhang Chen, Yanjun Fu,Qingzhong He, Lun Jiang, Jiangge Zheng, Yina Gao, Pinchao Mei, Zhongzhou Chen & Xueqin Ren, The amino-terminal structure of human fragile X mental retardation protein obtained using precipitant-immobilized imprinted polymers. Nature Communications, 2015, 6, 6634.
|
|
|
| Chinese
- Metal-free efficient photocatalyst for stable visible water splitting——Top ten major scientific progresses in China in 2015
- The nano-resolution imaging platform was awarded the first rate prize of Beijing Science and Technology in 2014
- Beamline 1W1 of BSRF started to runoperate in the couplingparasitic mode of BEPCII
- Synthesis of High Performance Polymer Materials for Field Effect-Transistors
- Surfactant molecular aggregates in green solvents
- GIXRD has played an important role in the characterization of organic thin-film transistors
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility