| Structure and Catalytic Activities of Ferrous Centers Confined on the Interface between Carbon Nanotubes and Humic acid |
| From: PublishDate:2016-06-03 Hits: |
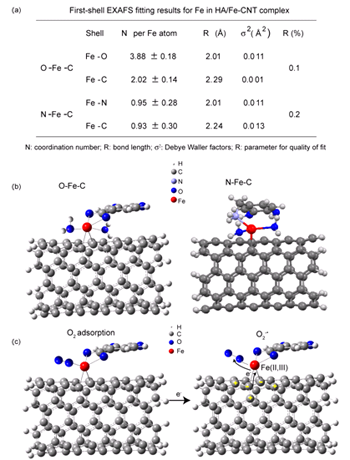
Preparation of heterogeneous catalysts with active ferrous centers is of great significance for industrial and environmental catalytic processes. Nanostructured carbon materials, which possess free flowing π electrons, can coordinate with transition metals, provide a confinement environment for catalysis, and act as potential supports or ligands to construct analogous complexes. However, designing such catalysts using NCM is still seldom studied to date. Herein, we synthesized a sandwich structured ternary complex via the coordination of Fe-loaded humic acid (HA) with C=C bonds in the aromatic rings of carbon nanotubes (CNTs), in which the O/N–Fe–C interface configuration provides the confinement environment for the ferrous sites. Their research has been published in Nanoscale, 2015, 7, 2651-2658. The team utilize Fe-loaded humic acid (HA) as a ligand to interact with CNTs and synthesize a ternary sandwich-type HA/Fe-CNT complex, which can confine the active ferrous centers at the interface between HA and CNTs. HA molecules possess rich oxygen/nitrogen-containing functional groups in the aromatic rings; thus, they can interact with CNTs via π–π stacking and provide coordination sites for iron cations as well. The synchrotron radiation X-ray absorption spectroscopy (XAS) studies reveal that the distorted octahedrally or tetrahedrally coordinated geometry contribute to the stabilization of ferrous sites. The quantitative analysis of XPS suggests that about 44.9% of Fe exists as Fe(II) species in the HA/Fe-CNT complex. The extended X-ray absorption fine structure (EXAFS) oscillations of Fe K-edge revealed the first shell of HA/Fe-CNT yields a Fe–O bond distance of 1.93 Å and Fe–C of 2.09 Å; moreover, the coordination numbers are 3.88 for Fe–O ( from two H2O molecule and one carboxylate group) and 2.02 for Fe–C, which correspond with the six-coordinated Fe model with four Fe–O and two Fe–C coordination. The C K-edge XANES spectra, a strong hybridization between CNT C π* and Fe 3d orbitals occurs, which is beneficial for charge redistribution between them. The density functional theory (DFT) calculation shows that the ternary system (HA/Fe-CNT) possesses higher chemical stability than binary systems (CNT-Fe or HA-Fe). The HA/Fe-CNT complex shows high catalytic activities for the activation of O2 and H2O2, which can be applied in the oxidative degradation of phenol red (PR) and bisphenol A (BPA) in aqueous media. Synchrotron radiation techniques provide crucial insights into the mechanism of O2 activation that the octahedrally or tetrahedrally coordinated geometry at Fe center and the strong hybridization between CNT C p* and Fe 3d orbitals induces the charge discretization of the aromatic rings on CNTs, which facilitates O2 adsorption and electron transfer from carbon to O2, thus enhances O2 activation. The reviewer evaluated that this work is a highly significant advance that is able to expand research into the carbon nanomaterials and other transitional metals. Moreover, HA is a naturally occurring organic substance; thus, the complex HA/Fe-CNTs can be obtained on a large scale using facile, economical, and green strategies.
Structural parameters of the HA/Fe-CNTs catalyst and the mechanism of O2activation. (a) The structural parameters and (b) geometry of HA/Fe-CNTs catalyst; (c) mechanism of O2activation by HA/Fe-CNTs Article: Structure and catalytic activities of ferrous centers confined on the interface between carbon nanotubes and humic acid. Bing Wang, Xiaoyan Zhou, Dongqi Wang, Jun-Jie Yin, Hanqing Chen, Xingfa Gao, Jing Zhang, Kurash Ibrahim, Zhifang Chai, Weiyue Feng* and Yuliang Zhao Nanoscale 7 (2015), 2651-2658. |
|
|
| Chinese
- Beamline 1W1 of BSRF started to runoperate in the couplingparasitic mode of BEPCII
- Synthesis of High Performance Polymer Materials for Field Effect-Transistors
- Surfactant molecular aggregates in green solvents
- GIXRD has played an important role in the characterization of organic thin-film transistors
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility