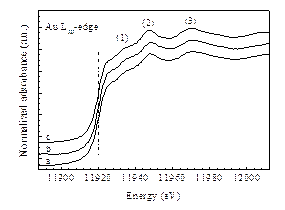| Morphologic effects of nano CeO2–TiO2 on the performance of Au/CeO2–TiO2 catalysts in low-temperature CO oxidation | ||||
| From: PublishDate:2015-06-16 Hits: | ||||
The catalytic oxidation of CO at low temperature has attracted considerable attentions because of its wide applications in the exhaust abatement of carbon dioxide lasers, automotive emission control, trace CO removal in enclosed atmospheres, CO preferential oxidation for proton exchange membrane fuel cells, etc. Among various heterogeneous catalysts involved, supported noble metals, e.g. Au, Pd, and Pt, have been proved catalytically effective for CO oxidation; meanwhile, it was also found that the activity of supported gold catalysts is strongly related to many factors like the gold-support interface, support geometry and quantum size effects. More recently, it was reported that the morphology/shape of nano-scale supports exhibits significant effect on the performance of gold catalysts. In this work, CeO2–TiO2 composite nanorods and nanoparticles were synthesized by hydrothermal and co-precipitation methods, respectively; with them as the supports, Au/CeO2–TiO2 catalysts were prepared by the colloidal deposition method with the aim to exclude the influence of gold species on their catalytic behavior and to get deep insight into the effect of support morphology on the performance of Au/CeO2–TiO2 catalysts. The results are newly published in Appl. Catal. B: Environ., 2014, 144(1): 498–506. N2 sorption, XRD, HRTEM, H2-TPR, XPS and XAS characterizations were used to clarify the relationship between the morphologic properties of CeO2–TiO2 and the catalytic performance of Au/CeO2–TiO2. HRTEM results show that Au/CeO2–TiO2 nanorods are dominated by CeO2 nanocrystals with (110) and (100) facets, while the Au/CeO2–TiO2 nanoparticles mainly expose (111) planes (Fig. 1). H2-TPR results indicate that the presence of Au strongly promotes the reduction of CeO2 in the Au/CeO2–TiO2 nanorods.
Fig. 1. TEM and HRTEM images of (a) and (b) Au/CeO2–TiO2-R, and (c) and (d) Au/CeO2–TiO2-P. Au LIII-edge X-ray absorption spectroscopy (XAS) measurements of the catalyst samples were performed at the beam line 1W1B of Beijing Synchrotron Radiation Facility (BSRF), Institute of High Energy Physics (IHEP), Chinese Academy of Sciences (CAS). Au LIII-edge XAS spectra were collected at room temperature in the fluorescence mode using a solid state detector. Fig. 2 shows the Au LIII-edge X-ray absorption near-edge structure (XANES) spectra of the Au/CeO2–TiO2-P and Au/CeO2–TiO2-R catalysts, together with Au foil as a reference. Gold foil (Au0) shows a shoulder at 11 933 eV as well as two intense peaks at 11 947 and 11 970 eV; the peaks at 11 933 and 11 947 eV are characteristic for pure gold f.c.c. structure ordered up to the third shell. It clearly illustrates that both Au/CeO2–TiO2-R and Au/CeO2–TiO2-P are identical to Au foil in their Au LIII-edge XANES spectra. Since XAS is a very sensitive technique in detecting Au cations, which gives a strong peak at 11921.6 eV in the Au LIII-edge XANES spectrum, the absence of this peak in the spectra of Au/CeO2–TiO2-R and Au/CeO2–TiO2-P further confirms the absence of Au cations in these catalysts, in well agreement with the XPS results.
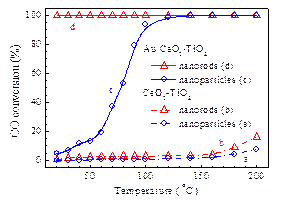
Fig. 2. XANES spectra of (a) Au/CeO2–TiO2-P, (b) Au/CeO2–TiO2-R, and (c) Au foil. The catalytic activity of Au/CeO2–TiO2 in CO oxidation is strongly related to the morphology of CeO2–TiO2; the CeO2-TiO2 nanorods as support endow the Au/CeO2–TiO2 catalyst with much higher activity than the CeO2–TiO2 nanoparticles (Fig. 3). The (110) and (100) facets of CeO2 dominated in the Au/CeO2–TiO2 nanorods are able to promote the oxygen migration and gold dispersion, which may then evidently enhance the redox capacity and catalytic activity of the Au/CeO2–TiO2 nanorods in CO oxidation at low temperature.
Fig. 3. Catalytic activities determined by the light-off tests in CO oxidation of (a) CeO2–TiO2-P, (b) CeO2–TiO2-R, (c) Au/CeO2–TiO2-P, and (d) Au/CeO2–TiO2-R. Information about this paper: Morphologic effects of nano CeO2–TiO2 on the performance of Au/CeO2–TiO2 catalysts in low-temperature CO oxidation. Appl. Catal. B: Environ., 2014, 144(1): 498–506. Shuna Li a,b, Huaqing Zhu a,*, Zhangfeng Qin a,*, Guofu Wang a, Yagang Zhang a,b, Zhiwei Wu a, Zhikai Li a,b, Gang Chen a,b, Weiwen Dong a,b, Zhonghua Wu c, Lirong Zheng c, Jing Zhang c, Tiandou Hu c, Jianguo Wang a,* a State Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, P.O. Box 165, Taiyuan, Shanxi 030001, PR China b University of Chinese Academy of Sciences, Beijing 100049, PR China c Institute of High Energy Physics, the Chinese Academy of Sciences, Beijing 100049, PR China E-mail addresses: qzhf@sxicc.ac.cn (Z. Qin); iccjgw@sxicc.ac.cn (J. Wang). |
||||
|
|
| Chinese
- Beamline 1W1 of BSRF started to runoperate in the couplingparasitic mode of BEPCII
- Synthesis of High Performance Polymer Materials for Field Effect-Transistors
- Surfactant molecular aggregates in green solvents
- GIXRD has played an important role in the characterization of organic thin-film transistors
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility