| Ce–Ti amorphous oxides for selective catalytic reduction of NO with NH3: Confirmation of Ce–O–Ti active sites |
| From: PublishDate:2013-06-15 Hits: |
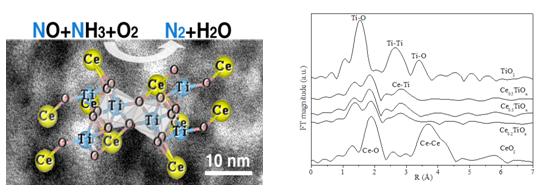
Nitrogen oxides (NOx) contribute much to acid rain, photochemical smog, and the depletion of tropospheric ozone. In China, the total amount of NOx emission has been controlled during the National Twelfth Five-Year Plan. The commercial technology for NOx control is the selective catalytic reduction (SCR) of NOx with NH3. Therein, the SCR catalyst is the most important. Previous studies have showed that a lot ofamorphous catalystspresent highactivity, and the working state of some crystalline catalysts is also amorphous. However, the component of the active site still cannot be confirmed completely.A team from School of Chemistry and Chemical engineering of University of Jinandesigned and prepared Ce–Ti amorphous composite oxides,andconducted studies on the NH3-SCR catalytic activity, the structure and the active sites. Their research has been published on August13th, 2012 in Environmental Science & Technology. The team found that Ce–Ti amorphous composite oxides can be obtained simply by adjusting the molar ratios of Ce/Ti using a coprecipitation method.Especially, Ce0.3TiOx (Ce/Ti molar ratio of 0.3) shows NOx conversion above 90 % from 175 to 400 °C and the N2 selectivity was always above 95 %.Importantly, the amorphous samples show higher activity than their crystalline equivalent at low temperatures. Moreover, the emergence of small CeO2 crystallites as for the crystalline sample is deleterious to activity.The results of temperature programmed reduction with H2 (H2–TPR),in situ FTIR spectra of NO adsorption and X-ray photoelectron spectroscopy (XPS) suggest the strong synergistic effect between Ce and Ti. Furthermore, X-ray absorption fine-structure (XAFS) confirmed the Ce–O–Ti short-range order structure, which is directly observed by field-emission TEM (FETEM).
As XAFS experiments (at BSRF) show the absence of the Ce–O–Ce structure in Ce–Ti amorphous composite oxides, the assignment of the activity of Ce0.3TiOx to the crystalline and amorphous CeO2 can be excluded. However, the detected Ti–O–Ti local structure (TiO2) shows much low activity to SCR reactions. Thus, the Ce–O–Ti short-range order structure is confirmed as the active site for the NH3-SCR reactions. This progress is reported entitled as “Research from University of Jinan Reveals New Findings on Environmental Science and Technology” by VerticalNews, American media of science and technology, in September 28th, 2012. http://www.verticalnews.com/premium_newsletters/Chemicals-and-Chemistry/2012-09-28/3133CH.html The research provides the scientific clues to understand the reaction mechanism of amorphous catalysts and develop new and highly active catalysts especially at low and medium temperatures. In this research, XAFS Station of Beijing Synchrotron Radiation Facility have helped the team to reveal the structure of the active site of the Ce–Ti amorphous composite oxide. “The short-range order structure is the smallest unit of the redox reaction in amorphous oxides, therefor, the research reported the phenomena similar to those of single-atom catalysis in metal catalysts in oxide cataltysts.However, whether or not it is suitable for other amorphous systems, further work is needed, in which XAFS must be a powerful tool”, explained by Zhaoliang Zhang, the team leader and the professor of School of Chemistry and Chemical engineering, University of Jinan.
Article: Ping Li, Ying Xin,Qian Li, Zhongpeng Wang, Zhaoliang Zhang*, Lirong Zheng, Ce–Ti amorphous oxides for selective catalytic reduction of NO with NH3: Confirmation of Ce–O–Ti active sites. Environmental Science & Technology, 46, 9600-9605, 2012. |
|
|
| Chinese
Science Highlights
Home /
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility