| lectrochemiluminescence Properties of [Pt2Ag4(C≡CC6H4R)8]n (R =CH3, n =1;R=H, n = 1 and 2) with Amine (TPrA and DBAE) as the Coreactant and Determination of Sudan I |
| From: PublishDate:2013-06-15 Hits: |
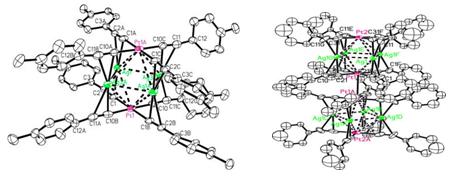
Two hexanuclear clusters, [Pt2Ag4(C≡CC6H4R)8](R=CH3 1;R=H 2), together with dimer [Pt2Ag4(C≡CC6H5)8]2 (3), have been synthesized by the self-assembly reaction and characterized by elemental analyses, electrospray ionization mass spectrometry, and 1H NMR spectroscopy and by X-ray crystallography for 1 and 3. Their research has been published on September 27th, 2012 in Inorganic Chemistry. It was reported that Forniés et al. tried many attempts at a complete X-ray study on the red dimmer crystal 3, but the crystal data were not good enough for a complete X-ray study. They only confirmed that two clusters [Pt2Ag4(C≡CC6H5)8] were linked by a Pt···Pt contact of 3.221(2) Å. Fortunately, crystals 1 and 3 were successfully characterized on a Mar CCD 165 nm diffractometer using the Beijing Synchrotron Radiation Facility with the 3W1A beam in this paper. Two crystallographically independent molecules A and B but with similar structural details were also found in 1. An ORTEP drawing of the molecule A structure of 1 is shown in Figure 1. The basic skeleton of 1 exhibits a distorted octahedral geometry of the metal centers with the platinum atoms in a mutually trans disposition and the four silver atoms in the equatorial plane. The Pt···Ag and Ag···Ag distances are shorter than the sum of the van der Waals radii, demonstrating the presence of strong Pt−Ag and Ag−Ag bonding interactions. The dimer 3 crystallizes in the tetragonal geometry of space group P4/nnc with two clusters 2 linked by a Pt···Pt contact of 3.2119(17) Å (shown in Figure 1), which is identical within experimental error to the analogous distance reported by Forniés et al. The two clusters are rotated with a torsion angle C21−Pt1−Pt1A−C21B of 48.7°, but within each of the clusters 2, the two square-planar [Pt(C≡CC6H5)4]2- fragments are found to be eclipsed. The Pt···Ag distances [3.1181(10) and 3.0957(11) Å] and Ag···Ag distance [3.0812(12) Å] are almost symmetrical, whereas the Ag−C bonds are asymmetrical, with Ag−Cα distances shorter than Ag−Cβ bonds (Δ = 0.174−0.233 Å) and comparable to those found in 1. ECL of 1 and 2 /amine systems in CH2ClCH2Cl was produced at the potentials of 1.14−1.19 V vs SCE, notably negatively shifted by about 0.38 V compared to those of the Ru(bpy)32+ / amine system. It is noted that Sudan I tends to decrease the ECL intensity of the 1 / DBAE system in CH2ClCH2Cl at a platinum working electrode. A new ECL method for the determination of Sudan I was developed with a linear range of 2.5 × 10−5 −1.0 × 10−3 M and a detection limit of 8.0 × 10−6 M. It is helpful for us to characterize by X-ray crystallography for 1 and 3 on a Mar CCD 165 nm diffractometer by the oscillation scan technique at 193 K using the Beijing Synchrotron Radiation Facility with a 3W1A beam. More studies of the functional heterometallic alkynyl complexes are in progress, and more brilliant synchrotron beams would surely help to examine crystals that we have already got.
Figure 1. Structure of cluster 1 (left) and 3 (right), measured on a Mar CCD 165 nm diffractometer by the oscillation scan technique at 193 K using the Beijing Synchrotron Radiation Facility with a 3W1A beam Article: Q.H. Wei,*L.J. Han,Y. Jiang,X.X Lin,Y.N. Duanand G.N. Chen*,Electrochemiluminescence Properties of [Pt2Ag4(C≡CC6H4R)8]n (R =CH3, n =1;R=H, n = 1 and 2) with Amine (TPrA and DBAE) as the Coreactant and Determination of Sudan I. Inorg. Chem., (51), 11117−11125, 2012. |
|
|
| Chinese
Science Highlights
Home /
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility