| Synthesis and electrochemiluminescence of a new water-soluble Re(I) complexes |
| From: PublishDate:2013-06-15 Hits: |
Re(I) tricarbonyl diimine complexes are characteristic with (MLCT) [dπ(Re) ®π*(N^N)], with rich photophysical and photochemical properties in visible light range, such as highly luminescence quantum yields, relatively long luminescence lifetimes, stable luminescence in visible light range and so on. Based on the advantages of Re(I) complexes, Re(I) tricarbonyl diimine complexes have been studied and applied by many researchers, but most of them were soluble in organic solvent, which limited the application of Re(I) complexes in analysis and bioanalysis. M.J. Li’s group from Fuzhou university synthesized a series of water soluble Re(I) tricarbonyl diimine complexes with good photophysical and photochemical properties by introducing hydrophilic groups into Re(I) complexes. The related study result was published in Dalton Trans in 2012. Study of introducing hydrophilic groups and acetonitrile into Re(I) complexes([Re(CO)3(L1)(AN)](PF6)2 and [Re(CO)3(L2)(AN)](PF6)3) showed the good water-solubility of these Re(I) complexes, while cyclic voltammetry study showed its good electrochemical properties. In addition, electrochemiluminescence of Re(I) complex/TPrA system and Re(I) complex/DBAE system indicating that the 3MLCT state was probably formed in both the Re(I) complexes.
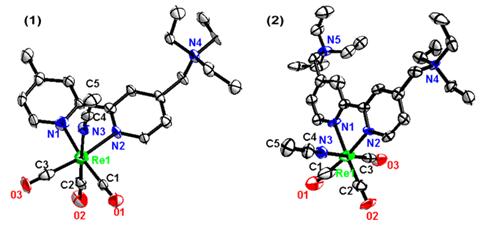
The solid state structures of [Re(CO)3(L1)(AN)](PF6)2 and [Re(CO)3(L2)(AN)](PF6)3 have been determined by X-ray crystallography using Beijing Synchrotron Radiation Facility with 3W1A beam (λ= 0.80 Å). Both complexes show distorted octahedral geometry with three carbonyl ligands groups being fac-oriented around the Re (I) center. The bond distances of Re-C and Re-N were typical of that found in rhenium(I) ricarbonyl diimine complexes. Angles subtended by the nitrogen atoms of ligand at the rhenium center N(2)-Re(1)-N(1) was 75.5°, which are smaller than the ideal angle of 90° adopted in octahedral geometry as required by the steric requirement of the chelating bipyridine ligand. The ECL of these complexesin the presence of co-reactant TPrA or DBAE in aqueous buffer were studied. Triton X-100 was found to strongly enhance the ECL intensity of the rhenium(I) complexes/TPrA system by about 190-fold. The results demonstrated that the new rhenium(I) carbonyl complexes show strong ECL signals in the presence of Triton X-100 in aqueous buffer, which would make it suitable for the potential analytical applications as ECL probes. Beijing Synchrotron Radiation Facility helped the group to solve the structures and provided powerful help to analyze the principle of the complexes. A lighter beam would be helpful for solving the structure of smaller crystals and developing of luminescent probes.
Article: Mei-Jin Li*, Xing-Liu, Yun-Qin Shi, Rui-Jia Xie, Qiao-Hua Wei, Guo-Nan Chen. Synthesis, structure, photophysics and electrochemiluminescence of Re(I) tricarbonyl complexes with cationic 2,2-bipyridyl ligands. Dalton Trans., 41, 10612–10618, 2012. |
|
|
| Chinese
Science Highlights
Home /
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility