Nanoceria - nanoparticles of cerium oxide (ceria), the most important rare earth nanomaterial - is well known as a high-performance catalyst because of the presence of mixed valence states of cerium and of oxygen vacancies. Recently, it has been reported that nanoceria has extraordinary antioxidant activity that makes it suitable as a therapeutic agent for several diseases including cancer, neurodegenerative diseases, diabetes and atherosclerosis, where reactive oxygen species act by impairing the normal redox balance. However, the inherent superoxide (SOD) scavenging ability has only been found in nanoceria with sizes of less than 5 nm, and bioactive nanoceria show very limited diversity with respect to shape.
LI Yuanyuan, HE Xiao and their colleagues from a research group led by Prof. ZHANG Zhiyong at the Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, Institute of High Energy Physics of the Chinese Academy of Sciences, collaborated with Prof. YIN Junjie of the US Food and Drug Administration to study the relationship between the physicochemical characteristics of nanoceria and its SOD scavenging ability.
The researchers found that the nanoceria they used, with a size of 5.1±0.4 nm, exhibited a negligible Ce3+/Ce4+ ratio and very low SOD mimetic activity. But by incubation with Cu, Zn-SOD or other electron donors, the SOD mimetic activity of nanoceria could be activated within minutes. The activity of the nanoceria could be regenerated again and again, or maintained for a long time when there was continuous electron transfer. This method will make it possible to develop nanoceria-based superoxide-scavengers with long-acting activity and tailorable characteristics. It has been proposed that this study opens the door to more practical and attractive biomedical applications for nanoceria.
The study, entitled “Acquired superoxide-scavenging ability of ceria nanoparticles”, has been published in Angew Chem Int Ed (DOI: 10.1002/ange.201410398) and selected as a Hot Topic in Surfaces and Interfaces by Wiley-VCH.
The group has been carrying out research on rare earth elements (REEs) since the early 1980s. Using neutron activation analysis, they have studied the contents and chemical species of REEs in biological and environmental samples, and evaluated physiological effects of REE on plants and neurotoxicity of REE on animals. The researchers developed a new high-quality gadolinium-loaded liquid scintillator for the Daya Bay Reactor Neutrino Experiment. This solved a major technical problem in neutrino detection and was regarded as one of the most important breakthroughs of the project. Recently, with the financial support of the National Natural Science Foundation of China and the Ministry of Science and Technology of China, they have focused on the environmental behavior and biological effects of rare earth nanomaterials. The distribution and fate of nanoceria in simulated aquatic ecosystems were investigated (Chemosphere, 2012, 89: 530-535; Nanoscale Res Lett, 2012, 7:84) and the impacts of nanoceria on various model animals such as Escherichia coli (Nanotoxicology, 2012, 6: 233-240; Environ Pollut, 2015, 196: 194-200), Caenorhabditis elegans (Environ Sci Technol, 2011, 45: 3725-3730), rats (Nanotechnology, 2010, 21: 285103) and mice (Int J Mol Sci, 2014, 15, 6072-6085) were assessed. They systematically studied the interactions between rare earth nanomaterials and plants (Chemosphere, 2010, 78: 273-279). For the first time, they reported that high-stability nanoceria can undergo biotransformation in plant systems and the heavy metal ions released in biotransformation play an important role in the phytotoxicity of metal based nanomaterials (ACS Nano, 2012, 6: 9943-9950; Nanotoxicology, 2011, 5: 743-753; Nanotoxicology, DOI: 10.3109/17435390.2014.921344; Nanotoxicology, DOI:10.3109/17435390.2013.855829; Environ Sci Technol, 2012, 46: 1834-1841; RSC Adv, 2015, 5: 4554-4560; Environ Pollut, 2015, 198: 8-14). Their research work has twice been published in Royal Society of Chemistry journals as cover articles (Metallomics, 2011, 3: 816-822; Environ Sci: Nano, 2014, 1: 459 – 465).
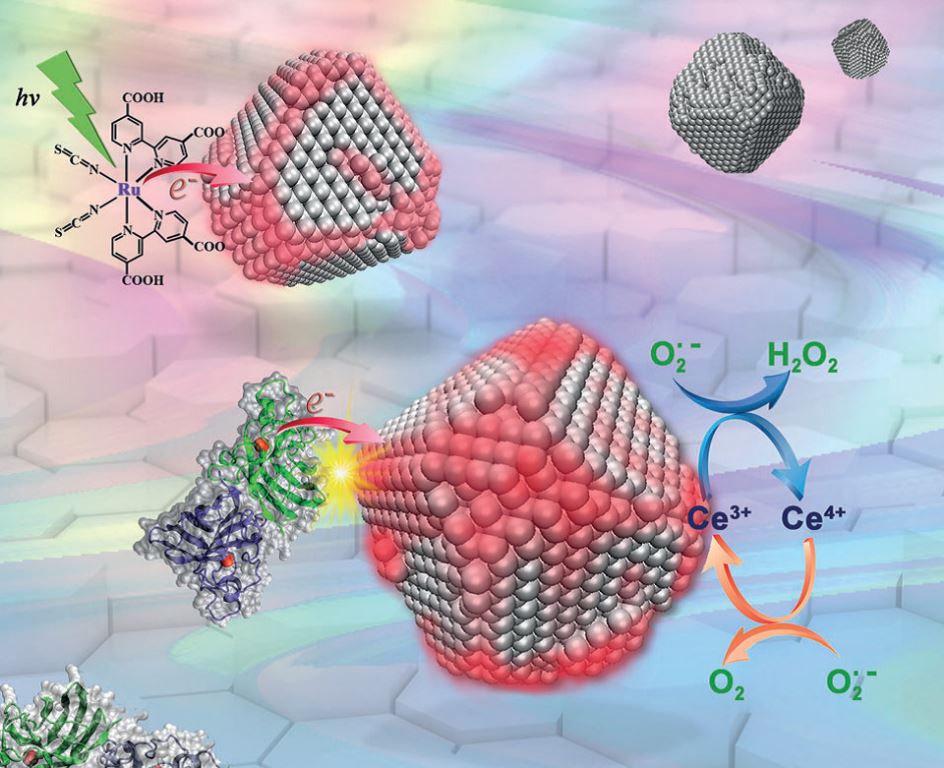
Nanoceria acquire superoxide scavenging ability after electron transfer (Image by IHEP)


